21st Century Medicine’s Aldehyde-Stabilized Cryopreservation
IMPORTANT NOTICE: This page provides an overview of the ASC method. We have completed survey evaluation electron microscope imaging of both a whole rabbit brain and whole pig brain that was preserved by this method and officially submitted to us for evaluation for the Brain Preservation Prize. Click the following image links to see these prize evaluation images sets:
Overview of Aldehyde Stabilized Cryopreservation (ASC):
In early 2015, 21st Century Medicine began a new set of experiments (spearheaded by recent MIT graduate Robert Louis McIntyre and with funding from the BPF) specifically designed to meet the requirements of our Brain Preservation Prize. 21CM’s ‘straight’ cryopreservation protocol had already shown that it was possible to perfuse a brain up to a sufficient concentration of cryoprotectant agent (CPA) to eliminate ice formation even when slowly lowering the brain’s temperature down to -130 degrees C for long-term storage. The key drawback of the technique being that in the process the brain is shrunken to half its original size due to osmotic dehydration (as discussed on a separate page) compressing neuronal processes at the ultrastructure level and making electron microscopic evaluation of its connectome virtually impossible.
It was reasoned that, instead of perfusing the living brain directly with CPA, it might be beneficial to first perfuse the brain with an aldehyde fixative followed by CPA perfusion later. Brains are routinely perfused with the fixative glutaraldehyde as the first step in the standard process of preparing brain tissue for electron microscopic evaluation. Glutaraldehyde is a crosslinking fixative that chemically reacts with some of the functional groups of proteins resulting in the creation of crosslinks which anchor these proteins to the cell’s cytoskeleton halting their normal enzymatic functioning. Within a few minutes of starting aldehyde perfusion almost all of the normal biological decay processes are stopped and after an hour of aldehyde perfusion the brain has been stabilized sufficiently such that it can sit for days at room temperature without significant change. The hope was that “aldehyde stabilizing” the brain in this manner would allow CPA perfusion to be performed under more optimal time and temperature conditions thus avoiding the osmotic dehydration and shrinkage.
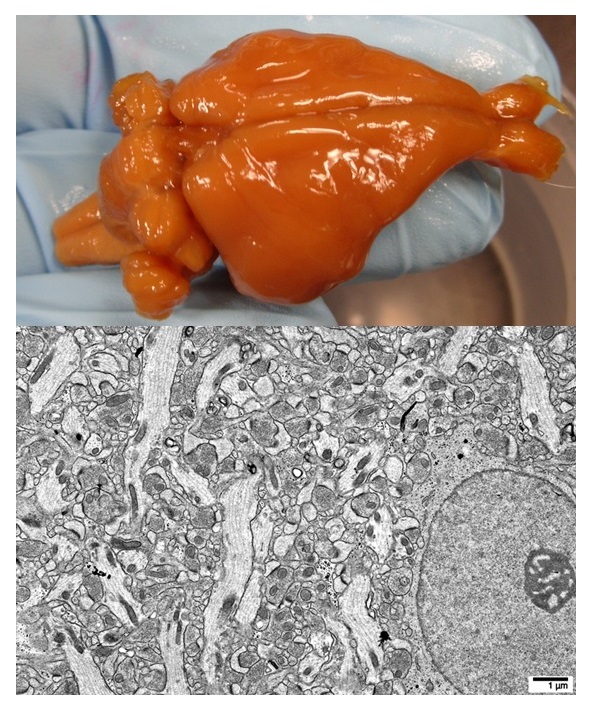
I am very happy to report that this reasoning seems to have been absolutely correct. The top image here is a whole rabbit brain which was first perfused with glutaraldehyde and then perfused near room temperature with a vitrifiable concentration of CPA. No gross shrinkage is evident. The bottom electron micrograph is from a similarly prepared rabbit brain that was stored intact in a freezer at -135 degrees C and then rewarmed. Vibrotome slices (200 microns thick) were taken through the brain’s hippocampus and CPA was washed out of the slices bringing them back to their aldehyde-fixed state allowing them to be prepared for standard EM imaging. The ultrastructure of this vitrified and -135 degrees C stored brain looks almost identical to aldehyde-only control brains. Although there are some minor fixation artifacts present, the electron micrographs look close to ‘textbook normal’.
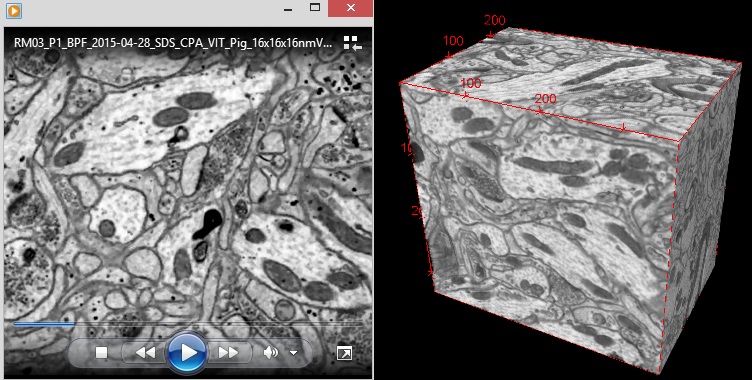
21CM has now tested this procedure on several whole rabbit and pig brains -each whole brain being perfused intact (in the skull) with glutaraldehyde followed by CPA. The whole brains were then stored in a freezer at -135 degrees C. The brains were rewarmed, CPA washed out, and selected regions were prepared for electron microscopic evaluation. 21CM has sent us many dozens of these electron micrographs and they show a stunning degree of ultrastructure preservation across the entire brain. They also sent us blocks of rabbit and pig brain tissue prepared by this method so that we could perform 3D EM imaging on them to directly test for traceability of the connectome. You can click the image of the video below to download a 3D stack of electron micrographs we imaged of a aldehyde stabilized cryopreserved pig brain sample they sent us:
21CM has already written these results into a scientific publication which has now been published in the Journal of Cryobiology. Here is a link to the open access publication:
http://www.sciencedirect.com/science/article/pii/S001122401500245X
Potential Advantages of the Aldehyde-Stabilized Cryopreservation (ASC) Procedure
This aldehyde-stabilized cryopreservation (ASC) technique has several potential advantages. ASC avoids the requirement of plastic embedding for long-term storage, meaning that, unlike Dr. Mikula’s protocol, no aggressive organic solvent step is necessary; lipids appear not to require further stabilization –thus there is no requirement for osmium, avoiding its expense and penetration issues entirely. But more importantly, this means that this aldehyde-stabilized cryopreservation would result in much fewer changes at the molecular level to brain tissue. Studies have long shown that although glutaraldehyde crosslinks proteins (stopping decay) it is quite good at preserving their sequential structure and positions within the cell. This is best shown in the recent paper “Mapping Synapses by Conjugate Light-Electron Array Tomography” (Collman, Buchanan, Phend, Micheva, Weinberg & Smith 2015) in which immunofluorescence is used to image the locations of a wide range of neurally important proteins in brain tissue that has been fixed with glutaraldehyde. While cold storage is still necessary, the fact that the brain is already aldehyde fixed means that even if a stored brain rewarmed for a few days no serious degradation would occur.
Aldehyde-stabilized cryopreservation may even have advantages over an optimized cryopreservation protocol. The initial perfusion of glutaraldehyde is almost instantly effective in stopping cellular decay and locks crucial molecules like ion channels and receptor proteins in place. This allows the subsequent CPA perfusion to be optimized (in terms of concentrations, temperature, and timing) to achieve the best possible structural preservation of the brain’s connectome.
It should be noted that aldehyde-stabilized cryopreservation has been suggested in the past. In fact its potential advantages were discussed extensively in chapter 9 of Eric Drexler’s 1987 book “Engines of Creation”.
To our knowledge, 21CM’s recent experiments are the first and only serious attempt to test this aldehyde-stabilized cryopreservation technique’s ability to preserve a whole mammalian brain.